Personal protective equipment (PPE) at work
About personal protective equipment (PPE)
Under health and safety legislation, employers have a legal duty to ensure suitable and sufficient risk assessments are carried out and adequate control measures are put in place to reduce the risk of harm to staff and patients, so far as is reasonably practicable. This includes identifying when PPE is required, and the type of PPE which should be used. PPE is designed to protect you from harmful substances in the course of your work such as chemicals or infectious agents.
The type of PPE you need will depend on a risk assessment, which should take into account the environment you work in and the procedures you carry out in relation to the risk present. PPE most commonly used by nursing staff includes gloves, aprons and respiratory protective equipment (RPE) such as FFP3 masks. The use of RPE requires users to be fit tested by a person competent to do so.
You can find out more in our respiratory risk assessment toolkit.
Remember, PPE is just one way of protecting staff at work. Education and training, workplace cleaning practices, ventilation, vaccination and employer's risk assessments for staff health also play an important role in managing the safety of staff and patients.
Please see Medicines and Healthcare products Regulatory Agency (MHRA) for detailed information on regulation relating to PPE.
Your employer should carry out a suitable and sufficient risk assessment which will identify if and when PPE is required for where you work, for example patients’ own homes. In some instances, for example when carrying out close contact care with a patient who may or is confirmed to have a respiratory infection such as influenza or COVID-19, you should be provided with a face fit-tested FFP3 mask and be given advice and training on face checking and donning and doffing procedures.
Your organisation should provide accessible information on this and on what the household can do to reduce risks, in advance of and during any visits.
Can I refuse to go into a persons’ home if I feel that it is unsafe?
Members may feel unsafe whilst delivering care in patient’s own homes for several reasons.
If you have concerns about other safety issues including personal safety, access to PPE and/or adequate ventilation, then you should follow the guidelines in our refusal to treat when making the decision. Contact us for further advice.
Additionally, if you have been verbally abused or threatened and you feel you will be at serious risk of assault if you enter the home, do not put yourself at risk. Call your manager and follow the RCN guidance on personal safety when working alone. Remember to report the incident using your organisation's incident reporting procedures as soon as possible.
Many health care professionals have impairments that could mean standard issue PPE is not effective. These include but are not limited to:
- sensory impairments
- use of prosthesis
- the use of mobility aids.
For some, PPE is a disabling barrier, for example, employees who communicate well through the ability to lip read will have this communication route disrupted if colleagues are wearing face masks.
Where there is a change in PPE requirements such as in response to an outbreak or national incident, novel issues can arise for health care professionals who have not previously been disabled at work. It is essential that employers' processes allow the opportunity for employees to discuss their specific needs regarding PPE and that they are supported by managers in this process.
The Equality Act 2010 (and in Northern Ireland the Disability Discrimination Act 1995) states that employers have a duty to make reasonable adjustments for employees who meet the definition of disabled. This applies to PPE equipment and the processes around administering PPE. The RCN believes that reasonable adjustments should be granted whether this definition is met or not, on the grounds that reasonable adjustments help us to work to the best of our abilities. The RCN’s advice guide on disability discrimination provides lots of helpful information on disability and your employer’s responsibilities.
The RCN expects that all employers support their staff to make known their needs in respect of PPE.
Your employer should work with you to ensure that any risk of PPE affecting your impairment and ability to continue in your role is recognised and processes put in place to mitigate the risk. This may mean adjusting processes around donning and doffing of PPE, exploring options for adapted PPE and/or opportunities to fit PPE, and be confident that it is fit for purpose prior to use in a clinical setting.
The RCN expects that line managers undertake a workplace risk assessment and refer to Occupational Health for further advice if appropriate. Where adjustments cannot be made, temporary redeployment to work that does not require PPE should be considered. You can also see our respiratory risk assessment toolkit.
Our publication Removing disabling barriers at work and our online guide on health ability passports cover the benefits of a diverse workforce including those with impairments and the reasonable adjustments process.
The Health and Safety Executive (HSE) also provides guidance for employers and employees on reasonable adjustments.
If the PPE provided to you is not fit for purpose (for example is dirty/contaminated or the elastic ties on the face masks has perished), you should:
- not use the equipment
- refer to your local policies on the use of PPE and report any quality issues immediately to managers, alongside completing a local incident form
- be provided with alternative PPE by your employer that is fit for use.
FFP3 face masks provide a higher level of respiratory protection than surgical face masks. Their effectiveness is dependent on the wearer undergoing a ‘fit’ test, to ensure a protective seal can be achieved.
There are two fit test methods, qualitative and quantitative. HSE’s guidance requires a fit test report/certificate to be made available to the employee to include the date, method and make and model of mask they have been fit tested for.
Fit tests must also be carried out whenever there is a change to the type or model of FFP3 mask or whenever there is a change in circumstances of the wearer that could alter the fit of the mask e.g. weight loss or gain or substantial dental work.
Fit testing must be carried out by a competent person as described by the Health and Safety Executive (HSE).
What if I have concerns about fit testing?
The RCN views any lack of competent fit testing as unacceptable. Employers should follow the HSE’s guidance on the knowledge requirements of a fit tester and the validation of equipment to be used.
Staff who are required to wear FFP3 masks should be trained in how to carry out a fit check in addition to donning and doffing training. A fit check must be carried out by the user every time an FFP3 mask is put on. The HSE has guidance on this including an instructional video.
To future proof FFP3 provision, the RCN supports innovative design of respiratory protection to ensure that there is a good selection of masks suitable for all, including black minority ethnic groups and female workers within the nursing workforce.
Where risk assessments require staff to wear FFP3 masks, all staff must have passed a face fit test for the mask being worn.
Further information
A list of external accredited fit testers can provide training for additional fit testers within your organisation.
Gloves should only be worn when indicated. RCN guidance Tools of the Trade provides specific guidance on glove use and skin care. It is recognised that wearing PPE for long periods - including gloves - can also create additional health, safety and wellbeing risks for nursing staff. PPE can be uncomfortable and potentially lead to heat stress, fatigue and heat related illness. Indications for glove use include contact with mucous membranes, blood and body fluids, chemicals or harmful drugs such as hormone creams or cytotoxic drugs. Gloves are not required for routine vaccinations including COVID-19 and influenza vaccines - see the RCN article Why you don’t always need gloves when giving vaccines.
When considering gloves members should also review if a plastic apron is required. Aprons should not be worn routinely and only if required.
Unnecessary glove/apron use and double gloving represent a waste of resources and may have implications for the skin/comfort of health care workers who wear these for long periods of time.
Double gloving
Double gloving is not required for care of patients with COVID-19 in any care setting. Please see your respective country national IPC guidance for further information.
It is recognised that wearing PPE for long periods can also create additional health and safety risks for nursing staff. PPE can be uncomfortable and potentially lead to heat stress, fatigue and heat related illness.
The RCN expects employers to meet their legal duties by taking all appropriate steps to both assess and mitigate the risk of nursing staff developing heat stress and related illnesses. These steps include:
- the workplace temperature: while there is no upper legal limit on workplace temperatures, the regulations state that workplace temperatures should be reasonable; the Chartered Institute of Building Engineers recommend that hospital environments should be 18 degrees centigrade.
- regular rest breaks during the shift: manufacturers’ recommendations on maximum time for wearing FFP3 face masks should always be followed (see RCN workplace risk assessment toolkit)
- allow staff to take power naps especially during night shifts
- access to comfortable rest facilities
- hydration/meal breaks
- access to toilet facilities
- raise awareness amongst staff of the signs of dehydration and heat stress and measures that can be taken to reduce the risk
- clear limits to not work beyond shift time and avoidance of continuous back-to-back long shifts. See our working time and breaks guidance.
The RCN also recognise that certain health conditions will make it more difficult to tolerate wearing PPE. Employers should assess any risks to these individuals and make necessary adjustments under both health and safety and equality law. See ‘PPE for staff with disabilities or impairments’ above. You can also see our Health ability passport guidance.
Further information
For information on how to reduce the risk of heat stress and related illness, please see Rest, Rehydrate and Refuel.
HSE have also produced a heat stress risk assessment toolkit for employers.
Our guidance on skin health is here along with our publication on maintaining skin health when using PPE.
PPE may contain natural rubber latex (NRL). Proteins found in NRL are known sensitizers which can cause allergic reactions in some individuals and may lead to serious health effects and should be avoided if possible.
Managing exposure and using safe alternatives
Whilst the use of PPE containing NRL has not been banned, however the Health and Safety Executive expect organisations to have systems in place to manage the risk of exposure to staff and patients with NRL allergies. Many health care organisations have taken steps to eliminate or severely restrict the use of products containing NRL from their sites.
The RCN expects all health and social care organisations to have and to follow their policies on the management of latex allergy in staff and patients.
Safer alternatives or latex free products should be used and staff/patients with known latex allergy should never be exposed to NRL-containing products. Powdered NRL gloves should never be used.
Where no safer alternatives are available and the risk of exposure to a biohazard exists, then a COSHH risk assessment will inform whether the use of PPE containing NRL is acceptable for use by staff without NRL allergy.
Incident reporting
Any allergic-type reactions to PPE, including skin rashes, should be reported using the organisations incident reporting form and through the yellow card reporting system to the MHRA.
Organisations that have no alternative supplies to NRL PPE should document this as a Serious Untoward/Adverse Incident.
Read more about raising concerns about PPE.Wearing PPE can be uncomfortable and can lead to heat stress, fatigue and heat-related illness. It is recognised that wearing PPE for long periods can also create additional health and safety risks for staff.
For those experiencing symptoms due to menopause, this can sometimes add to the burden of working in intensive areas of practice.
It is always important to recognise that for many reasons the impact of the menopause may differ greatly for individuals.
Symptoms can be compounded when having to wear PPE, especially for long periods of time, and it is important that staff are supported to enable them to cope in these challenging times.
It is important to remain hydrated, to use a good hydrating moisturiser which can be applied under masks and to keep some cool wet wipes handy. You can find further guidance in our Reproductive health and menopause at work advice guide.
Please also see our advice on heat stress above.
Further information
The RCN has lots of helpful clinical information at:
For information on how to reduce the risk of heat stress and related illness, please see Rest, Rehydrate and Refuel. HSE have also produced a heat stress risk assessment toolkit for employers
Menopause at work: managing hot flushes and PPE (a Nursing Standard article which may require you to log in to access in full)
RCN guidance on skin health and Maintaining skin health when using PPE may also be useful.
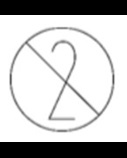
Single use PPE should only be used once. It is identified through this symbol present on packaging or equipment instructions.
Please see our guidance on skin health and our publication maintaining skin health when using PPE.
Respiratory risk assessment toolkit
Look at our respiratory risk assessment toolkit which supports RCN members and wider health care professionals manage risk in the workplace.
Raising concerns about PPE
Organisations must have effective procedures in place to allow nursing staff and their representatives to raise any concerns in relation to equipment, policies and processes for managing COVID-19 at the earliest opportunity.
Nursing staff should feel able to raise concerns without detriment and should receive timely feedback on their concerns. If your concerns remain unresolved, refer to:
and speak to your line manager.
If you have followed these steps and the issue is still not resolved, please contact us.
Winter wellbeing
Sickness
Page last updated - 23/02/2026





